How Many Electrons Are Transferred in H2o
The remaining two-carbon fragment is oxidized and the electrons transferred to NAD storing energy in the form of NADH. Atoms have outer electrons with the same princi.
How Many Electrons Are In H2o Quora
View Answer On January 1 2015 ABC.
. This pattern of reaction is referred to as Markovinkov addition after the person 1 who first discovered that HBr adds in this way to a double bond. B This arrangement enables the plant to absorb light energy of a variety of wavelengths. Coenzyme A CoA is attached via its sulfur atom to the two-carbon intermediate forming acetyl CoA.
A Excited electrons must pass through several pigments before they can be transferred to electron acceptors of the electron transport chain. Acid solution 2H 2e- H2 gas aerated water O2 2H2O 4e- 4OH- Sealed Source. One of the most famous reactions of these Fe IV O-based complexes is OAT in which the oxygen in Fe IV O is transferred to the substrate orbital with lone-pair electrons 2932.
Electrons are transferred from one atom to another atom. The electrons generated from the oxidation process mustb t f db t ft be transferred become part of another reaction known as the reduction reaction. C They enable the plant to absorb more photons from light energy all of which are at the same wavelength.
The intermediate carbocation is the tertiary carbocation rather than the primary carbocation that would be produced by addition to the CH 2 end of the double bond. From the co-enzyme channel electrons are funneled to molecular oxygen though chain of e- carrier they include flavoproteins Fe-SUQ Co Q cytochromes in respiratory chain e- are transferred in 3 ways 1As e- s 2as H-atoms3. The fundamental concept of this theory is that when an acid and a base react with each other the acid forms its conjugate base and the base forms its conjugate acid.
We can classify many reagents as combinations of electrophile and. The BrønstedLowry theory also called proton theory of acids and bases is an acidbase reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. As hydride ions which bears two electrons during the flow of e-every members of ETC get Alternately reduced and.
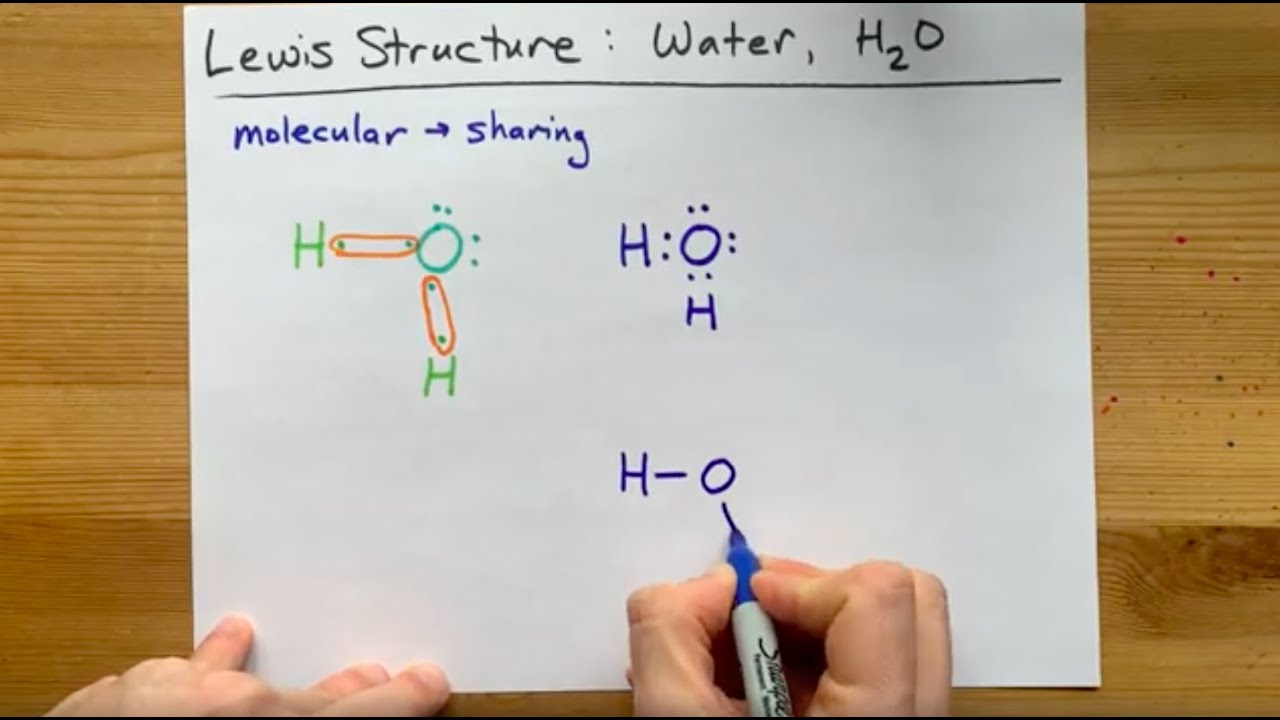
Lewis Structure Of H2o Water Dihydrogen Monoxide Youtube

A Electron Charge Transfer Between Water Molecules In The Formation Download Scientific Diagram

Reduction Of O2 To H2o And Its Free Radical Intermediates A Lewis Download Scientific Diagram
No comments for "How Many Electrons Are Transferred in H2o"
Post a Comment